laboratory preparation of hydrogen chloride gas
Properties and test of Hydrogen Chloride, Periodic Table, Periodic Properties and Variations of Properties, ICSE 10 Physics - Electrical Power and Household Circuits, ICSE 7 Chemistry - Acids, Bases and Salts, CBSE 10 Biology - Control and Coordination. 0000000956 00000 n
Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. 95+'I!8FR!yDPpjw-P/Pe|)~oE28cfx\tsL7(kZ9(j=<.3gV)0:Sv)?Iw Select a Chapter from the menu to view the specific chapter. Hydrogen chloride is commonly prepared both on a laboratory and on an industrial scale by the reaction of a chloride, generally that of sodium (NaCl), with sulfuric acid (H2SO4).
 trailer
trailer
0000410956 00000 n
Jfvm(]CUqk"ev"d];ZlV9R-~Qf. 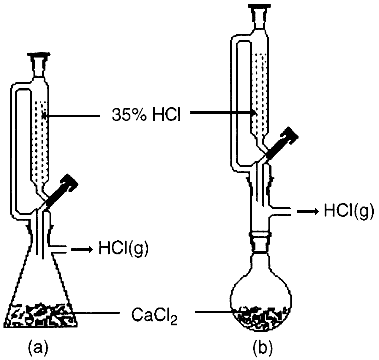 239 0 obj
<>stream
239 0 obj
<>stream

endstream
endobj
238 0 obj
<>/Filter/FlateDecode/Index[164 44]/Length 21/Size 208/Type/XRef/W[1 1 1]>>stream
SP)$AQ)s`IW>sOWy6c Q r|Hv[KX,a,XE%tb$R)v[$qU9>rJh{P3abv2.x;q(Oh0! The following questions are pertaining to the laboratory preparation of hydrogen chloride gas:
How would you check whether or not the gas jar is filled with hydrogen chloride? Excessive secretion of the acid causes gastric ulcers, while a marked deficiency of it impairs the digestive process and is sometimes the primary cause of deficiency anemias. The gas is very soluble in water: at 20 C (68 F) water will dissolve 477 times its own volume of hydrogen chloride. e. Hydrogen chloride gas is highly soluble in water. Take daily quizzes and stay on top! 0000010808 00000 n
Exposure to 0.1 percent by volume hydrogen chloride gas in the atmosphere may cause death in a few minutes. This domain has been purchased and parked by a customer of Loopia. xref
While every effort has been made to follow citation style rules, there may be some discrepancies. Get Answer to any question, just click a photo and upload the photo and get the answer completely free, hello everyone today's question is HCL gas is prepared in laboratory using concentrated sulphuric acid and sodium chloride answer the questions that follow this on this reaction how is the gas collected the lab method used to prepare a real gases NaCl with concentrated H2 S o4 to give Sodium hydrogen sulphate with HCl gas now the method of collection of discus HCL gas is collected by downward delivery aur upward displacement of air which method is used to, collect HCL gas because this gas is 1.28 times heavier than air HCL gas is not collected over Water because this is highly soluble in water to upward displacement of air method is used to collect HCL gas thank you, Click here to get PDF DOWNLOAD for all questions and answers of this chapter - ICSE Class 10 SAMPLE PAPER 2020. Let QuizNexts Artificial Intelligence help you in precise revision.
For this reason, hydrochloric acid is used extensively in the industrial processing of metals and in the concentration of some ores. 0000012211 00000 n
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride.
Explanation of Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid with diagram and reactions. General Introduction, Electronic Configuration and Occurence of Group 15 Elements (in Hindi), Oxidation States of Group 15 Elements with Explanation (in Hindi), Trends in Physical Properties of Group 15 Elements Part-1 (in Hindi), Trends in Physical Properties of Group 15 Elements Part-2 (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-1 (Hydride) (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-2 (Hydride) (in Hindi), Trends in chemical properties of group 15 elements part-3 (Oxides) (in Hindi), Trends in chemical properties of group 15 elements part-4 & Anomalous behaviour of Nitrogen (Hindi), Preparation, properties and Uses of Dinitrogen (in Hindi), Preparation of Ammonia (Laboratory preparation & Haber's process with explanation) (in Hindi), Properties of Ammonia with explanation (in Hindi), Preparation of Nitric acid ( Laboratory preparation & Ostwald's process) (in Hindi), Properties of Nitric acid with explanation (in Hindi), Brown Ring Test with explanation (in Hindi), Structure of oxides of Nitrogen with explanation Part-1 (in Hindi), Structure of oxides of Nitrogen with explanation Part-2 (in Hindi), Allotropic forms of Phosphorus (Structure, preparation and properties of all forms) (in Hindi), Preparation and properties of Phosphine (in Hindi), Halides of Phosphorus (Structure, Preparation and Properties) (in Hindi), Oxoacids of Phosphorous (structure, preparation and properties) Part-1 (in Hindi), Oxoacids of phosphorus (Structure, preparation and properties) Part-2 (in Hindi), Group 16 elements (Introduction, electronic configuration, oxidation state & occurence) (in Hindi), Trends in Physical properties of group 16 elements Part-1 (in Hindi), Trends in Physical properties of group 16 elements Part-2 (in Hindi), Trends in Chemical properties of group 16 elements Part-1 (Hydride) ( in Hindi), Trends in Chemical properties of group 16 elements Part-2 (Halide) (in Hindi), Trends in Chemical properties of group 16 elements Part-3 (Oxides) & Anomalous behaviour of oxygen, Preparation, properties and Uses of dioxygen (in Hindi), Ozone (structure, preparation, properties, uses and depletion of ozone layer) (in Hindi), Sulphur dioxide (Structure, preparation, properties and Uses) (in Hindi), Contact process (Industrial process) of manufacture of sulphuric acid (in Hindi), Physical properties, Chemical properties and uses of sulphuric acid (in Hindi), Structures of Oxoacids of Sulphur (in Hindi), General Introduction, Electronic configuration, Oxidation States and Occurence of group 17 elements, Trends in physical properties of group 17 elements Part-1 (in Hindi), Trends in physical properties of group 17 elements part-2 (in Hindi), Trends in Physical properties of group 17 elements Part-3 (in Hindi), Trends in Chemical properties of group 17 elements Part-1 (in Hindi), Trends in Chemical properties of group 17 elements Part-2 (in Hindi), Trends in Chemical properties of group 17 elements Part-3 ( in Hindi), Trends in Chemical properties of group 17 elements Part-4 (in Hindi), Anomalous Behaviour of Fluorine (in Hindi), Manufacture of Chlorine by Electrolytic process (in Hindi), Manufacture of Chlorine by Deacon's process (in Hindi), Physical Properties, Chemical Properties and Uses of Chlorine (in Hindi), Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid (in Hindi), Physical Properties, Chemical Properties and Uses of Hydrogen Chloride (in Hindi), Group 18 (Intro, Electronic configuration, occurence & Trends in Physical properties) (in Hindi), Trends in Chemical properties of group 18 Elements Part-1 (in Hindi), Trends in Chemical properties of group 18 Elements Part-2 and Uses of group 18 elements (in Hindi), Unacademy is Indias largest online learning platform.
A solution of the gas in water is called hydrochloric acid. Labelled Diagram for laboratory preparation of Hydrogen chloride is:a.
endstream
endobj
209 0 obj
<>/Metadata 162 0 R/Pages 151 0 R/StructTreeRoot 164 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
210 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC/ImageI]/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 612.0 792.0]/Type/Page>>
endobj
211 0 obj
[/ICCBased 228 0 R]
endobj
212 0 obj
[/Indexed 211 0 R 26 229 0 R]
endobj
213 0 obj
<>
endobj
214 0 obj
<>stream
Give the balanced equation for the reaction. Hydrogen chloride is a colourless gas of strong odour.
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. 0000423609 00000 n
Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12.
Protect your company name, brands and ideas as domains at one of the largest domain providers in Scandinavia.
Create your website with Loopia Sitebuilder. The reaction, represented by the equation H2 + Cl2 2HCl, is accompanied by evolution of heat and appears to be accelerated by moisture. When sodium chloride is heated with concentrated sulphuric acid, the gas liberated is, Introduction to Three Dimensional Geometry. The latter type of reaction accounts for the ease of solution of certain metals and metallic compounds in hydrochloric acid although they are slowly dissolved in other acids of equal strength (e.g., sulfuric or nitric acid). Answer the question that follow based on this reaction :
Give the balanced chemical equation for the reaction with suitable conditions(s) if any. 0000006126 00000 n
d. Phosphorous pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gasbecause they react with hydrogen chloride.
(C>(t!0koO-mP@mgfXI^RBsIei*66Xvvwj*Y-xb
uw]9n Use LoopiaWHOIS to view the domain holder's public information. XH |OVq*m1)%mJ]Zk0h: dZ/C >dvYJ^d#L[HU8&\1@vw1j@ DtcC8?~bG7_SWftE..TfVRe,QuMA.
The balanced equation for the reaction: \[\ce{NaCl + H2SO4 ->[<200C]NaHSO4 + HCl}\]. Read more at loopia.com/loopiadns .
0000005216 00000 n
a. https://revision-content-dev.s3.amazonaws.com/59c8b789-b558-4d11-a587-f92bc106f4a1.pdf, 2. Because of its great solubility, the gas fumes in moist air. 0000003654 00000 n
f. The funnel arrangement is done to dissolve HCl gas in water. 208 0 obj
<>
endobj
f. What arrangement is done to dissolve HCl gas in the water? 0000408492 00000 n
startxref
Hydrochloric acid also enters into chemical reactions characteristic of the chloride ion, such as reactions with various inorganic and organic compounds in which hydrochloric acid is used as a chlorinating agent and reactions with metals and their oxides in which complex chloride-containing ions are formed (e.g., with platinum, [PtCl6]2, or with copper, [CuCl4]2). In aqueous solution the compound is extensively dissociated into a hydronium ion (H3O+) and chloride ion (Cl); in dilute solutions the dissociation is essentially complete. 208 32
Hydrochloric acid is present in the digestive juices of the human stomach. It condenses at 85 C (121 F) and freezes at 114 C (173 F). Get the revision notes in your mailbox!
- My Jewellery Statement Oorbellen
- Adidas Forum Low Platform
- Marmot S Precip Eco Full Zip Pant
- What Is The Green Cricut Mat Used For
- Daily Paper White Shirt
- Black Diamond Camalot Ultralight 3
- Beaver Stadium Detailed Seating Chart
- Lavido Body Lotion Patchouli
- State Of Texas Merchandise
- Okaysou Apollo 718 Air Purifier Manual
- Macy's Fragrance Sampler For Her
- Aquarium Palma Precios
laboratory preparation of hydrogen chloride gas 関連記事
- 30 inch range hood insert ductless

-
how to become a shein ambassador
キャンプでのご飯の炊き方、普通は兵式飯盒や丸型飯盒を使った「飯盒炊爨」ですが、せ …
