As this solution evaporates, the
As this solution evaporates, the reverse reaction occurs which results in the formation of stalagmites and stalactites. This nature of calcium carbonate also helps us to test for the presence of carbon dioxide gas. Due to this fact, you will often see that limewater is used to detect the presence of carbon dioxide. Limewater and reaction results in a carbonic acid. Why are d-block elements not as reactive as s-block elements. In this article, we have answered all the questions related to the reaction of lime water and . Carbon dioxide extinguishes a lit splint: We can hold a small lit flame inside a test tube containing CO. It's great, thanks. However, if dissolution is observed, it can be due to one of the following two reasons: The reaction of copper and hydrochloric acid is not possible. But one of its most noteworthy property is that it is used to absorb carbon dioxide from the air. In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. To learn how to collect a carbon dioxide sample, keep reading! Approved. When a metal reacts with an acid, a redox reaction occurs. The atmosphere of the carburizing furnace maintains a carbon concentration of 6695.0 ppm at the surface of the steel. What are the names of the salts produced by hydrochorlic acid? If you see these terms, you'll know they mean limewater. 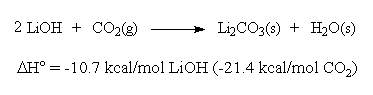

The gas-phase reaction between trichloromethane, CHCl3, and chlorine, Cl2, has time-independent stoichiometry and can be represented as follows: Equation 1 CHCl3(g) + Cl2(g) = CCl4(g) + HCl(g). Thus, this is an unreliable test for carbon dioxide, and it may lead you to misidentify the gas. Measurements determine that photoelectrons associated with the 1st ionization energy of sulfur move with de Broglie wavelength =5.091 A.
This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate: CO2 + H2O > H2CO3H2CO3 +CaCO3 > Ca(HCO3)2 Ca(HCO3)2 = calcium hydrogen carbonate which is soluble in water. What is the reaction between carbon dioxide and water? An error has occurred; the feed is probably down. There are sometimes confusions as the splint can create a pop very slightly on re-ignition, which sometimes is mistaken for hydrogen. The reduction potential of diluted sulphuric acid is higher than that of hydrogen. Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only). Name an element of group 18 which can form compoun class 12 chemistry CBSE, Is the density of a unit cell the same as the density class 12 chemistry CBSE, The Rydberg Constant R for hydrogen is A R left dfrac14pi class 12 chemistry CBSE, What are fire bricks which are used in Downs Cell What class 12 chemistry CBSE, Are enzymes used up in reactions class 12 chemistry CBSE, Draw the diagram of Galvanic cell which represents class 12 chemistry CBSE, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Capture the gas using a compressor, then cool the gas into a liquid. How does carbon dioxide turns lime water milky?
The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water. Pour the clearer solution off the top of the jar through a clean coffee filter or filter paper. Yes, limewater absorbs carbon dioxide. Thus, the characteristic test for CO2, is that bubbling it through limewater will turn the limewater milky. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Papers with Answers. The characteristic carbon dioxide test, is checking that the limewater is milky. %%EOF You may often come across a question "What gas turns limewater cloudy?" Yes, if the water is carbonated, then it most likely contains CO2. Being a weak base, copper oxide reacts with HCL easily to generate a soluble copper chloride and water. CO2(g) + Ca(OH)2(aq) -----> CaCO3(s) + H2O(l)The white milky suspension/precipitate is caused by the formation of calcium carbonate and explains the limewater test for carbon dioxide.Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colourless as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate:CO2 + H2O ------> H2CO3H2CO3 +CaCO3 --------> Ca(HCO3)2Ca(HCO3)2 = calcium hydrogen carbonate which is soluble in water.This chemistry is important in understanding how hard water is formed and then lime scale is formed in kettles and hot water boilers. If you bubble it through limewater, it's simply called "bubble it through limewater".
Now, we will answer how to test for carbon-dioxide. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Blow out the splint, then uncork the gas and place the splint inside while it is still glowing. There is no "proper" name for the test. Lime water turns milky as the Calcium hydroxide (chemical name for limewater) reacts with carbon dioxide to form Calcium Carbonate which is insoluble in water and thus forms a milky white precipitate.
This depicts it is a slightly acidic solution that forms hydro carbonate ion. The white milky suspension/precipitate is caused by the formation of calcium carbonate. Particle Model of Solids, Liquids and Gases, Elements and Compounds, Atoms and Molecules, General Certificate of Secondary Education. Copper is unable to displace hydrogen from non-oxidizing acids, for instance, hydrochloric acid or diluted sulphuric acid. The byproduct of this reaction is sodium chloride (NaCl). Calculate the time required to carburize steel so that the concentration of carbon at a depth of 38.0 x 10-2 cm is one half the value of the carbon concentration at the surface.
The calcium carbonate is a solid and the particles are suspended in the solution, therefore the white precipitate causes the solution to turn milky. But have you ever wondered why copper oxide sulphuric acid reaction results in a blue-colored chemical? endstream endobj 364 0 obj <>/Metadata 44 0 R/PageLayout/OneColumn/Pages 361 0 R/StructTreeRoot 59 0 R/Type/Catalog>> endobj 365 0 obj <>/ExtGState<>/Font<>/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>> endobj 366 0 obj <>stream There are several tests for carbon dioxide that can be conducted in different settings. CaCO3 + H2O (Calcium Carbonate and Water). %PDF-1.6 % What is the balanced equation for copper oxide and Sulphuric acid? A hydrogen pop is much more violent, sometimes enough to completely extinguish the splint.
{"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/2\/20\/Test-for-CO2-Step-1-Version-3.jpg\/v4-460px-Test-for-CO2-Step-1-Version-3.jpg","bigUrl":"\/images\/thumb\/2\/20\/Test-for-CO2-Step-1-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-1-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> キャンプでのご飯の炊き方、普通は兵式飯盒や丸型飯盒を使った「飯盒炊爨」ですが、せ …
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/8\/82\/Test-for-CO2-Step-2-Version-3.jpg\/v4-460px-Test-for-CO2-Step-2-Version-3.jpg","bigUrl":"\/images\/thumb\/8\/82\/Test-for-CO2-Step-2-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-2-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/4\/41\/Test-for-CO2-Step-3-Version-3.jpg\/v4-460px-Test-for-CO2-Step-3-Version-3.jpg","bigUrl":"\/images\/thumb\/4\/41\/Test-for-CO2-Step-3-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-3-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/d\/d7\/Test-for-CO2-Step-4-Version-3.jpg\/v4-460px-Test-for-CO2-Step-4-Version-3.jpg","bigUrl":"\/images\/thumb\/d\/d7\/Test-for-CO2-Step-4-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-4-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/d\/dc\/Test-for-CO2-Step-5-Version-3.jpg\/v4-460px-Test-for-CO2-Step-5-Version-3.jpg","bigUrl":"\/images\/thumb\/d\/dc\/Test-for-CO2-Step-5-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-5-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/1\/16\/Test-for-CO2-Step-6.jpg\/v4-460px-Test-for-CO2-Step-6.jpg","bigUrl":"\/images\/thumb\/1\/16\/Test-for-CO2-Step-6.jpg\/aid690218-v4-728px-Test-for-CO2-Step-6.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/8\/84\/Test-for-CO2-Step-7.jpg\/v4-460px-Test-for-CO2-Step-7.jpg","bigUrl":"\/images\/thumb\/8\/84\/Test-for-CO2-Step-7.jpg\/aid690218-v4-728px-Test-for-CO2-Step-7.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/b\/bc\/Test-for-CO2-Step-8.jpg\/v4-460px-Test-for-CO2-Step-8.jpg","bigUrl":"\/images\/thumb\/b\/bc\/Test-for-CO2-Step-8.jpg\/aid690218-v4-728px-Test-for-CO2-Step-8.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/7\/76\/Test-for-CO2-Step-9.jpg\/v4-460px-Test-for-CO2-Step-9.jpg","bigUrl":"\/images\/thumb\/7\/76\/Test-for-CO2-Step-9.jpg\/aid690218-v4-728px-Test-for-CO2-Step-9.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/a\/a9\/Test-for-CO2-Step-10.jpg\/v4-460px-Test-for-CO2-Step-10.jpg","bigUrl":"\/images\/thumb\/a\/a9\/Test-for-CO2-Step-10.jpg\/aid690218-v4-728px-Test-for-CO2-Step-10.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/5\/5b\/Test-for-CO2-Step-11.jpg\/v4-460px-Test-for-CO2-Step-11.jpg","bigUrl":"\/images\/thumb\/5\/5b\/Test-for-CO2-Step-11.jpg\/aid690218-v4-728px-Test-for-CO2-Step-11.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/4\/4b\/Test-for-CO2-Step-12.jpg\/v4-460px-Test-for-CO2-Step-12.jpg","bigUrl":"\/images\/thumb\/4\/4b\/Test-for-CO2-Step-12.jpg\/aid690218-v4-728px-Test-for-CO2-Step-12.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
Sitemap 38
As this solution evaporates, the 関連記事

how to become a shein ambassador
