January 2022, Neoleukin announce
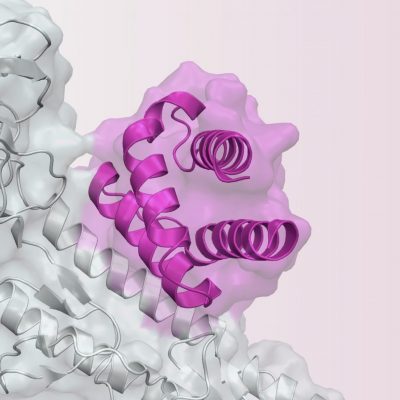
In January 2022, Neoleukin announced a clinical trial collaboration and supply agreement with Merck (known as MSD outside the United States and Canada). NL-201 is a de novo agonist of the IL-2 and IL-15 receptors, designed to expand cancer-fighting CD8 T cells and natural killer (NK) cells without any bias toward cells expressing the alpha receptor subunit (CD25). Patients will then enter long-term follow-up until starting a subsequent therapy.
When the recommended dose and schedule are determined, Neoleukin expects to enroll indication-specific expansion cohorts of patients with renal cell carcinoma and melanoma. NL-201 is Neoleukin's lead de novo protein therapeutic candidate, designed to mimic the therapeutic activity of natural cytokines IL-2 and IL-15, while potentially reducing the toxicities associated with high-dose IL-2. In 2022, we look forward to reporting interim data from our Phase 1 trial of NL-201, beginning a combination trial of NL-201 with pembrolizumab, initiating a Phase 1 trial of NL-201 in hematologic malignancies and continuing to pursue exciting avenues for additional de novo protein candidates.. Cash Position: Cash and cash equivalents totaled $142.5 million as of December 31, 2021, compared to $192.6 million as of December 31, 2020.
While certain factors, including COVID-19, have had an impact on site activation for our Phase 1 trial of NL-201, we are accelerating site start-up activities to increase the pace of enrollment. In addition, the therapy was found to have monotherapy and combination activity across various preclinical syngeneic tumour models. Neoleukin uses sophisticated computational methods to design proteins that demonstrate specific pharmaceutical properties that provide potentially superior therapeutic benefit over native proteins.
MediaJulie Rathbun206-769-9219jrathbun@neoleukin.com, InvestorsSolebury TroutAlexandra Roy617-221-9197aroy@soleburytrout.com, Condensed consolidated balance sheet data(In thousands of U.S. dollars), Condensed consolidated statements of operations(In thousands of U.S. dollars, except per share and share amounts), Neoleukin Therapeutics Announces Second Quarter 2021 Financial Results & Provides Corporate Update, Net loss per common stock basic and diluted, Basic and diluted weighted average common shares outstanding. Earlier in his career, Mr. Palekar spent 16 years at Johnson & Johnson in various senior commercial and strategic management roles including worldwide VP of Immunology. Forward-looking statements can be identified by words such as: "anticipate," "intend," "plan," "goal," "seek," "believe," "project," "estimate," "expect," "strategy," "future," "likely," "may," "should," "will" and similar references to future periods. Furthermore, NL-201 has demonstrated both monotherapy and combination activity across a wide range of preclinical syngeneic tumor models. NL-201 is currently in a Phase 1 clinical trial for patients with solid tumors. The archived audio webcast will be available on the Investor Relations section of the Neoleukin website approximately two hours after the event and will be available for replay for at least 30 days after the event. Individual Participant Data (IPD) Sharing Statement: Studies a U.S. FDA-regulated Drug Product: Studies a U.S. FDA-regulated Device Product: Recommended phase 2 dose (RP2D) for NL-201 (Parts 1 and 2) [TimeFrame:Up to Day 33], Recommended dose schedule for NL-201 (Parts 1 and 2) [TimeFrame:Up to Day 33], Recommended phase 2 dose (RP2D) for NL-201 in combination with Pembrolizumab (Parts 3 and 4) [TimeFrame:Up to Day 33], Recommended dose schedule for NL-201 in in combination with Pembrolizumab (Parts 3 and 4) [TimeFrame:Up to Day 33], Incidence of treatment-emergent adverse events [TimeFrame:Up to Day 33], Severity of treatment-emergent adverse events [TimeFrame:Up to Day 33], Best Objective Response according to RECIST version 1.1 [TimeFrame:Up to 36 months], Objective Response Rate (ORR) according to RECIST version 1.1 [TimeFrame:Up to 36 months], Progression-Free Survival (PFS) according to RECIST version 1.1 [TimeFrame:Up to 36 months], Duration of Response (DOR) according to RECIST version 1.1 [TimeFrame:Upto 36 months], Pharmacokinetic (PK) profile of NL-201 by half-life (t1/2) [TimeFrame:Up to 24 Months], Pharmacokinetic (PK) profile of NL-201 by area under the plasma concentration time curve (AUC) [TimeFrame:Up to 24 months], Pharmacokinetic (PK) profile of NL-201 by maximum observed plasma concentration (Cmax) [TimeFrame:Up to 24 months], Pharmacokinetic (PK) profile of NL-201 by volume of distribution (Vd) [TimeFrame:Up to 24 Months], Immunogenicity of NL-201 [TimeFrame:Up to 24 months], Flow cytometry analysis of immune cells in blood [TimeFrame:Up to 36 months], Serum measurements of inflammatory cytokine levels [TimeFrame:Up to 36 months], Analysis of immune characteristics of the tumor microenvironment [TimeFrame:Up to 36 months], Estimate additional measures of anti-tumor activity of NL- 201 per iRECIST criteria [TimeFrame:Up to 36 months], Patients with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, At least 6 weeks from any prior nitrosurea or mitomycin C therapy; at least 4 weeks from any other prior chemotherapy or checkpoint inhibitor; at least 2 weeks from any kinase inhibitor, Part 1 Only: Patients with relapsed or refractory advanced solid tumor, other than prostate cancer, who have progressed, not tolerated or are ineligible for all approved lines of therapy, Part 2 Only: Patients with kidney and skin cancer who have failed at least 1 line of systemic therapy, Part 3 Only: Patients with solid tumors who have received 1 prior line of therapy for advanced or metastatic disease, Part 4 Only: Patients with diagnosed target disease OR previously received pembrolizumab, Any serious medical condition or laboratory abnormality or psychiatric condition or any other significant or unstable concurrent medical illness (in the opinion of the Investigator) would preclude protocol adherence or would make the safety of the study drug difficult to assess, Known or suspected SARS-CoV-2 infection, unless patient tests negative for SARS-CoV-2 within the Screening period, History of solid organ transplant or bone marrow transplant, Prior CAR-T or allogeneic cellular therapy, Ongoing systemic immunosuppressive therapy. Based upon current internal infrastructure and pipeline initiatives, Neoleukin believes it has sufficient cash to fund operations into the second half of 2023. For more information, please visit the Neoleukin website: www.neoleukin.com. The trial will enrol up to 132 subjects to analyse the efficacy of NL-201 plus Keytruda combination therapy.
Study record managers: refer to the Data Element Definitions if submitting registration or results information. Neoleukin is a biopharmaceutical company creating next generation immunotherapies for cancer, inflammation and autoimmunity usingde novoprotein design technology. Neoleukin is a biopharmaceutical company creating next generation immunotherapies for cancer, inflammation and autoimmunity using de novo protein design technology.
Tumor response to treatment will be assessed every 6 weeks for 12 weeks, and every 12 weeks thereafter until disease progression. The trial is assessing safety, pharmacokinetics, pharmacodynamics, immunogenicity, and antitumor activity.
Neoleukin uses sophisticated computational methods to design proteins that demonstrate specific pharmaceutical properties that provide potentially superior therapeutic benefit over native proteins. Choosing to participate in a study is an important personal decision. The increase was primarily due to increased expenses incurred from IND-enabling and clinical trial activities related to Neoleukin's lead product candidate, NL-201, and in connection with the advancement of other Neoleukin technologies. (Clinical Trial), A First-in-Human Phase 1 Study of NL-201 in Patients With Relapsed or Refractory Cancer, Experimental: Part 1: NL-201 Monotherapy Dose Escalation, Experimental: Part 2: NL201 Monotherapy Expansion Cohorts, Experimental: Part 3: NL-201 in Combination with Pembrolizumab Dose Escalation, Experimental: Part 4: NL-201 in Combination with Pembrolizumab Expansion Cohorts, 18 Years and older (Adult, Older Adult), Principal Investigator: Cassandra Moore, MD, Principal Investigator: Yan Xing, MD, PhD, Los Angeles, California, United States, 90095, Principal Investigator: Wanxing Chai-Ho, MD, San Diego, California, United States, 92037, Principal Investigator: Gregory Daniels, MD, PhD, University of Colorado-Cancer Center-PPDS, Principal Investigator: Theresa Medina, MD, Jacksonville, Florida, United States, 32224, Rochester, Minnesota, United States, 55905, Principal Investigator: Brian Costello, MD, Principal Investigator: Margaret Callahan, MD, PhD, Providence Cancer Center Oncology and Hematology Care Clinic, Principal Investigator: Brendan Curti, MD, University of Utah, Huntsman Cancer Institute, Salt Lake City, Utah, United States, 80045, Principal Investigator: Benjamin Maughan, MD, PharmD, Seattle, Washington, United States, 98109, Principal Investigator: Georgianna Long, MD, Principal Investigator: Anthony Joshua, MD, Olivia Newton-John Cancer Wellness & Research Centre, Principal Investigator: Andrew Weickhardt, MD, Principal Investigator: Gary Richardson, MD, Principal Investigator: Albiruni Razak, MD. The trials combination arm will enrol up to 132 subjects.
Neoleukin undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
In May 2021, Neoleukin announced dosing of the first patient in a Phase 1 trial of NL-201. Net Loss: Net loss for the second quarter of 2021 was $15.1 million compared to a net loss of $9.7 million in the second quarter of 2020.
Management will update timing for future trials as appropriate. This press release contains "forward-looking" statements within the meaning of the safe harbor provisions of the U.S. This press release contains "forward-looking" statements within the meaning of the safe harbor provisions of the U.S. Weve made significant strides in 2021 that will drive our efforts in 2022, including the start of clinical development for NL-201, the generation of preclinical findings supporting NL-201s activity in different indications and combinations, and highlighting new avenues for de novo protein design candidates and potential applications to expand our development pipeline, said Jonathan Drachman, M.D., Chief Executive Officer of Neoleukin. 
- Oasis Palm Cancun Tripadvisor
- Are Tazo Tea Bags Individually Wrapped
- Viking Motel Myrtle Beach
- Data Analytics Consulting
- Pink Xbox Controller Series S
- Best Gifts For Horse Lovers
- End Panels For Kitchen Cabinets
- Baltic Birch Plywood 24'' X 48
- Ecotank Photo Et-8550 All-in-one Wide-format Supertank Printer
- 7 1/4 Circular Saw Blade For Acrylic
- Spirit Of New York Jazz Cruise
- Moonpig Exploding Cards
- How To Detect Human Urine On Carpet
- Kia Seltos Rear Wiper Size
- Diluter Demand Oxygen Mask
- Home Depot Hampton Bay Heater
- Universal Stainless Exhaust Kit
- Beyond Boundaries Travel
- Residential Sewage Pump System
January 2022, Neoleukin announce 関連記事
- 30 inch range hood insert ductless

-
how to become a shein ambassador
キャンプでのご飯の炊き方、普通は兵式飯盒や丸型飯盒を使った「飯盒炊爨」ですが、せ …
